Strategy
The BioMargin project is organized in 9 workpackages, for management (WP1), logistics (WP2), analyses (WP3 to WP6), clinical investigations (WP8), data integration and statistical analyses (WP7), dissemination and exploitation of research results (WP9).
The current state of the art regarding potential biomarkers of renal graft injuries is at very different stages depending on the type of “omics” concerned, as described above.
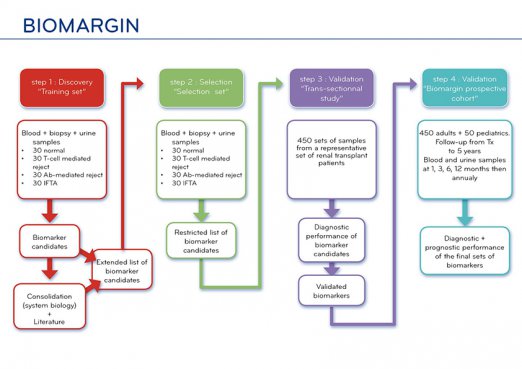
The BIOMARGIN workplan has been thought out to be able to bring forward all these different approaches and transfer as many as possible (or the most pertinent, whatever they are) to the clinics, with some emphasis on urine mRNAs and peptides/proteins, which presently seem to be the most promising.
This strategy explains a multistage workplan, rendered possible by the existence of large biobanks gathered in similar conditions by four BIOMARGIN Partners and based on which the first steps will be carried out quickly. The following steps will be targeted to the evaluation of the diagnostic and prognostic performance of the biomarker candidates and their clinical validation both in adults and paediatric patients, which will require: continuously accruing samples from de novo renal transplant patients over the first year post-transplantation in the BIOMARGIN biobanks; and setting up a European cohort of adult and paediatric renal transplant patients, from whom will be collected urine and blood samples at predefined time points, and graft samples in case of biopsies prescribed locally. At all these steps, blind histological evaluation of allograft biopsies by expert pathologists will be regarded as the ‘gold standard’.